Crash Course on Covd-19 Vaccines in Development
So, what’s the DL on Covid-19 Vaccines? Here’s a mini crash course for non-science folx.
Given my education in clinical pharmacology and a fairly decent knowledge of cancer immunotherapies, I have been asked a lot of very good questions on the vaccines currently in development for Covid-19. There are so many vaccines currently in development, each with their own MOA (mechanism of action). This is my attempt to simplify the information in a way that is accessible to non-science folx.
 Image: Fusion Medical Animation via Unsplash
Image: Fusion Medical Animation via Unsplash
??? ?? ??? ????-???-? (??? ????? ???? ?????? ?????-??) ???????? ?????? ???? ???????????? ?????????
Conventional vaccines use small amounts of live or inactivated disease causing organisms, there are a few currently in development for Covid-19 (Sinovac, Sanofi+GSK).
A few of the SARS-CoV-2 vaccines are an RNA vaccine (eg: Moderna¹, Pfizer²), which instead of infecting with the actual disease causing organism, it injects messenger RNA (mRNA) that codes for that disease causing antigen. So the idea is that because of this, you will see less adverse effects (because you’re not actually injecting the virus in this case) and is much easier and quicker to manufacture, especially in large scale.
The other type of vaccine currently in development is actually an adenovirus (which is a fairly mild virus that causes minor cold and GI symptoms) that is re-engineered so it produces the DNA for the Sars-Cov-2 spike proteins (eg: Oxford³, CanSino⁴). So it’s different than the mRNA, but again, we anticipate lower adverse effects because you are beginning with an adenovirus instead of the actual Covid-19 causing virus. Adenoviruses have been used as a vector against other viruses and even cancer.
Moderna, Oxford and CanSino are currently recruiting for the Phase 2/3 studies and are the ones that are most advanced in development. Pfizer has recently completed it’s Phase 1 and entering Phase 2/3 shortly.
There are also a few other in earlier phases of development. These include vaccines that inject synthetic DNA that codes for the disease causing antigen (eg: Inovio⁵), others that use other viruses such as recombinant vesicular stomatitis virus similar to the Ebola vaccine (Merck⁶), a modified measles vector platform (Merck⁶) and dozens of others still in the preclinical phase.
In addition, and out of the scope of this Q&A, there are also antiviral and monoclonal antibodies (mAbs) in development in parallel as a ????????? for Covid-19.
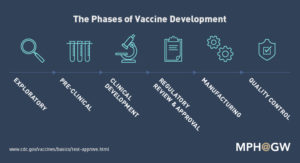
Created by MPH at the George Washington University
???? ?? ??? ???? ??????? ???? ?????
So far the side effects from Phase 2 studies of the Moderna, Oxford, Pfizer and CanSino vaccines seem to be your typical vaccine like — injection site reactions, fever, fatigue, headache, muscle aches and chills. These are essentially signs of an immune response. However, both are relatively novel in terms of delivery. We definitely need longer terms immunogenicity studies to discern any of the side effects that are rare and would require larger sample sizes, but so far the safety profile looks promising. Importantly, we haven’t seen a signal for ADE (Antibody Dependent Enhancement) in the clinical trials so far (see below for more on this); however the numbers treated in the Phase 1 studies are small.
???? ????? ?????? ?? ????????? ??? ????????? ????????????
Whereas the Phase 1/2 studies were generally limited to under age 55, the Phase 2/3 studies of most vaccines have in fact include the elderly population (including above age 70). These studies will give us additional information on the efficacy and safety concerns in the elderly populations.
Pediatric studies have not been done as yet. The Phase 1/2 studies excluded individuals under age 18. Often dosing schedules look different in the pediatric population, with many vaccines requiring an extra dose or two in the younger. Hopefully as we get data on safety from the Phase 2/3 studies, pediatric studies will be initiated. This is why it would be important for as many to get the vaccine when it is made available to us, so we are able to protect the vulnerable in our society.
?? ????? ? ??????? ???? ???????? ????????? ??????????? ???? ??? ?????????
Antibody dependent enhancement (ADE or viral induced enhanced disease) is not uncommon with vaccines against viral agents. We have seen it occur with early iterations of several vaccines, including influenza. It is also why many of the MERS vaccine candidates weren’t successful or why we don’t have a widely available vaccine against RSV. It is super critical to do intensive, comprehensive studies looking especially at a variety of dosages to see what the antibody response is and whether there is a possibility of enhancing antibodies (worsening) in addition to neutralizing antibodies (protective). So far we haven’t seen this in the trials for the Covid-19 vaccines in the lead, but this is why the Phase 3 studies with 20–30k patients is extremely important because that is where we will discern this more accurately. This is incredibly important to ensure the vaccine is completely safe, and for all subgroups.
???????? ???????? ??????? ??????? ????????? ?? ????????????
The studies on natural infection with SARS-CoV-2 that have looked only at antibodies are missing the memory B and T cell response (these are also a part of a body’s immune response), which is the other half of the adaptive immunity equation. In fact, T-cell response is especially important in responses to viral infections.
In vaccine development, you always want an immune response stronger than that with a natural infection. Data from Phase 1/2 studies from Moderna, Oxford, CanSino and Pfizer vaccines have shown a durable and sustained immune response that includes both neutralizing antibodies as well as a robust T-cell immune response. So far data has been collected to around 3 months out, but data from the next Phase of studies should give us information on longer term immunity.
??? ????? ??? ??????? ?? ??????
Too early to tell, but most likely a prime-boost regimen. A minimum of a booster shot will be required at least 28 days after the first dose. This is not dissimilar to, say, influenza where a child receiving a flu shot for the first time receives the first dose that essentially primes, and the second dose that offers the immunity. Likely the SARS-CoV-2 vaccine will need to be given annually, but we do not know that yet.
??? ???? ??? ??????? ?? ????????????
Good question. This is something organizations such as the CDC’s ACIP (Advisory Committee on Immunization Practices) and the equivalent in other countries are working on to create a set of criteria that allows for equitable access to the vaccine in terms of priority, within each country and also, importantly, globally.
The general idea with the vaccines against SARS-Cov-2 is that there will be priority that goes to the high risk groups, specifically the elderly, health care and other front line and essential workers as well as long term care homes, shelters, prisons etc.
Remember that this also adds to the extra real-world safety data of the vaccines, so by the time the vaccine is released for the general public, there is a lot more patient years worth of safety data available.
???? ????? ??????? ???? ?????????
A study recently came out of Spain, a country that was very hard hit with Covid-19. It showed that despite how wide spread the infection was, only 5% of their population were seropositive⁷. In Sweden, despite no lockdowns, it was only 7.3%⁸. At this point, it is immensely clear that herd immunity through natural infection is unattainable, unethical and will cause significant collateral damage.
Also, I cannot think of any other disease where herd immunity was achieved without vaccination. It is simply not possible, at least not without significant collateral damage.
?? ??-????????? ?????????
Typically immunity against coronaviruses are short-lived; however, we have seen longer immunity from SARS and MERS vs the common cold coronavirus, so it is possible for there to be some immunity from a natural infection of SARS-CoV-2. However, we do not know how long this would last, and whether there are any clinically meaningful differences in immunity between those who have mild vs moderate vs severe disease. So far, there are no accurate reports of re-infection. What we have found is leftover viral debris that is picked up by a PCR, as it is not able to differentiate between infectious vs non-infectious viral matter.
???? ?? ??? ????? ???????? ????? ??? ??????? ????? ?????
Viruses tend to replicate very quickly, and in doing so, are messy and sloppy. Mutations are essentially mistakes that occur in the genome when a virus replicates. That said, in general, coronaviruses mutate much slower compared to say, influenza. So far, the mutations that have occurred have had limited meaningful effect on the core behavior of the virus. The new variant appears to be slightly more infectious⁹, but has not resulted in any worse outcomes¹⁰. Importantly, the new variant was neutralized by convalescent plasma (old variant) with similar efficacy¹¹. This tells us that the vaccines in development currently should still be effective against the new strain.
Of course, there is always a possibility that between now and the release of the vaccine, Sars-CoV-2 further mutates. But hopefully the variations do not cause any major changes in the virus, and continue to be minor in nature.
??? ??? ????????? ????? ??? ????? ?? ??????????? ?? ????? ?????????
The amount of collaboration and pooling of resources on Covid-19 has never been seen before! It’s incredible. Also, many had already initiated development of these for the original SARS, so it didn’t take too much effort for them to pivot, thus saving time.
Importantly, Phase 3 trials generally are event driven — and as we know about event driven studies, these cannot be shortened in duration or sped up. We have shrunk the development as much as we can, reduced some of the bureaucracies and also prioritized Covid-19 reviews and processes at the governmental and institutional level. So all of these have contributed to the possibility of expedited timelines.
????? ? ??? ??? ??????? ???? ?? ?? ???? ?????????? ?? ???, ????? ????
Absolutely, without a doubt, I would be in line to receive this vaccine. As to which, data will tell us if there is a lead candidate and what global availability of each vaccine would look like. I have absolute confidence in the vigour and robustness that goes into vaccine development, and I have full faith in science.
Signed,
Unambiguous Science
— — — — — — — — — — — — — — — — — — — — — — — — — — — — — —
References:
1- https://www.nejm.org/doi/full/10.1056/NEJMoa2022483?query=featured_home
2- https://www.medrxiv.org/content/10.1101/2020.08.17.20176651v1
3- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31604-4/fulltext
4- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31605-6/fulltext
5- http://ir.inovio.com/news-releases/news-releases-details/2020/INOVIO-Announces-Positive-Interim-Phase-1-Data-For-INO-4800-Vaccine-for-COVID-19/default.aspx
6- https://www.statnews.com/2020/05/26/merck-aims-to-begin-human-tests-of-two-different-covid-19-vaccines-this-year/
7- https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31483-5/fulltext
8- https://www.theguardian.com/world/2020/may/21/just-7-per-cent-of-stockholm-had-covid-19-antibodies-by-end-of-april-study-sweden-coronavirus
9- https://www.biorxiv.org/content/10.1101/2020.06.12.148726v1
10- https://www.cell.com/cell/pdf/S0092-8674(20)30820-5.pdf?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867420308205%3Fshowall%3Dtrue
11- https://www.biorxiv.org/content/10.1101/2020.06.20.161323v2
